
Most user-friendly LMS
SkyPrep has an easy-to-use and customizable interface that customers love.

FDA 21 CFR Part 11 focuses on the security and confidentiality of customer information, administration of e-documents, and the approval requirements for e-documents and signatures.
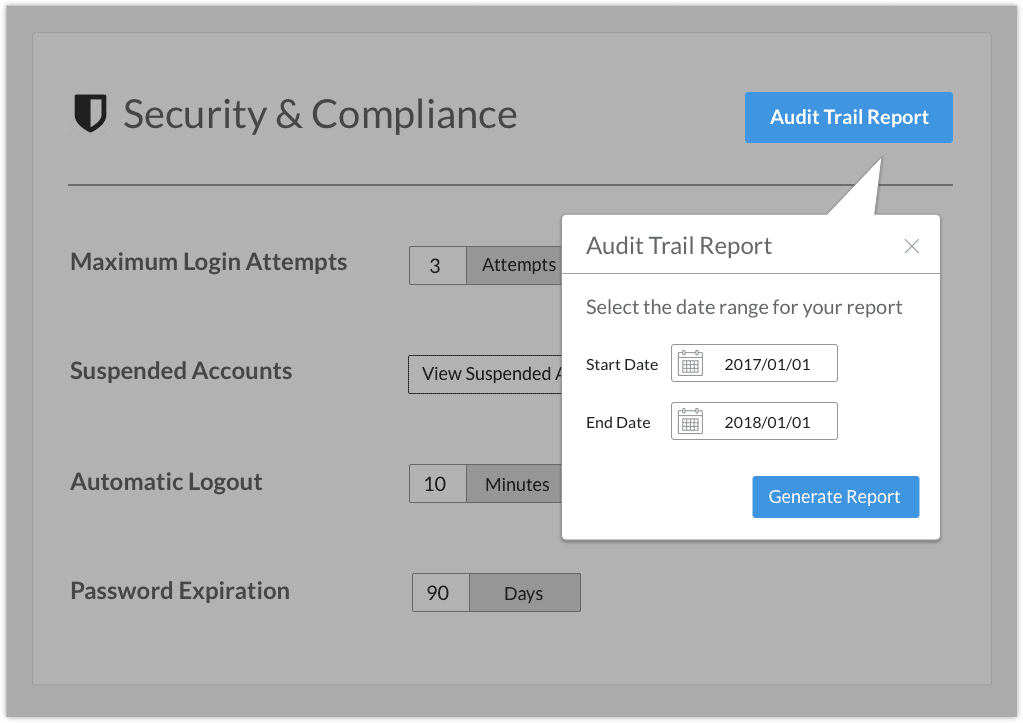
Audit trail reports provide a read-only record of any updates pertaining to system-critical objects. Every audit trail report specifies the event, date/time, affected data object, operator identification, type of change, and comments.
For audit trail documentation, course versioning is a necessity. Any changes made in SkyPrep are stored without altering the previously stored records, making it possible to see previously recorded information.
SkyPrep offers configurable electronic signature options that enable adding a full user name, unique ID, date, time as well as meaning of the signature when it’s captured.
Admins are instantly notified with inactivity lockout and restricted login attempt features. Access rights to different utilities are achieved by configuring different permissions and roles.
Authentication in the SkyPrep LMS require a user ID and password. All users are assigned unique user IDs that make it possible to track the actions of every user.
SkyPrep uses an ISO27001 compliant Amazon AWS hosting service and provides consistent backups of customer information. The user data is protected by SSL.

SkyPrep has an easy-to-use and customizable interface that customers love.

Our team provides all of the necessary onboarding, training and help resources. The exceptional customer support team is always ready to assist users.

SkyPrep keeps all training records updated in real-time for hands-on compliance reporting.

SkyPrep provides audit and compliance reports, including a “Record of Learning” for each user, that offers a full historical transcript of learning for that user.